iCare probes are safe to use
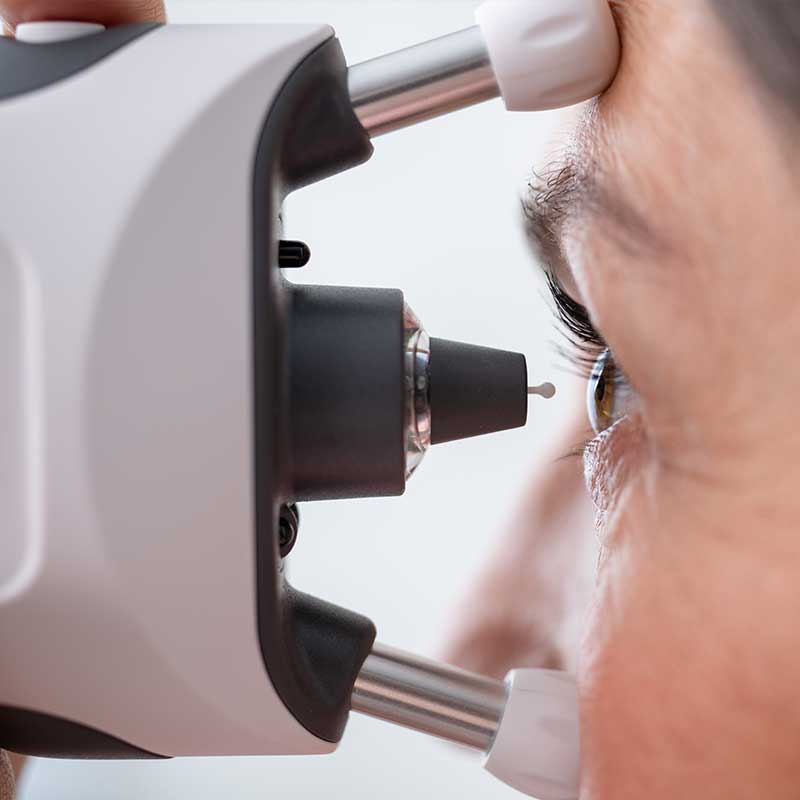
Measure eye pressure safely without air puff, anesthetic drops or other preparation. iCare tonometers work on a rebound principle, in which a lightweight probe moves in a magnetic field and makes momentary contact with the cornea.
iCare TP022 probes are individually packed in sterile blisters and delivered in 20 pcs and in 50 pcs packages. The probes are intended for single use only and should not be cleaned or reused. Cleaning or mishandling the probe can damage and shorten the lifetime of the device. Reusing probes could cause cross contamination of virus or bacteria and it may shorten the life cycle of the device. Using the original iCare probes is critical for ensuring patient safety and the accuracy of IOP measurements.
iCare probes for iCare tonometers
Accuracy And Repeatability Result
The probes are integral to accurate functioning of iCare tonometers. They are subject to a rigorous design and development process to ensure that every IOP measurement is accurate and reliable. The probes maintain stringent weight, length, and straightness tolerances to ensure consistent IOP measurements. The manufacturing and usage of medical devices are highly regulated. The accuracy and repeatability of iCare tonometers are validated only with genuine iCare probes.
Efficient And Painless Measurements
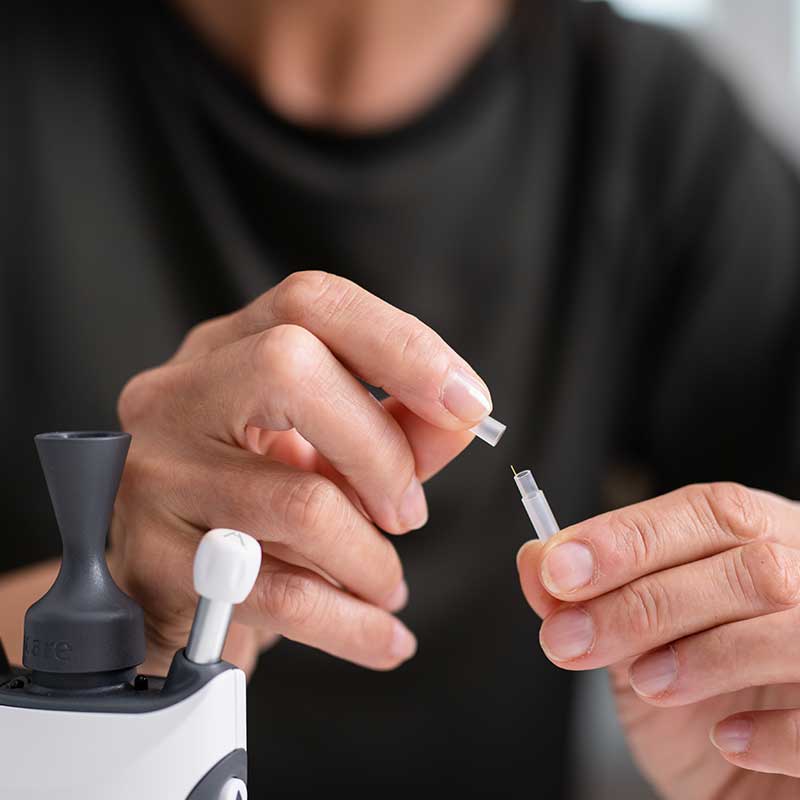
During the IOP measurement, these probes make quick, gentle touches to the cornea during the measurement. In all cases, the process is painless. The probes are designed for easy insertion and removal, attaching magnetically to the tonometer for a secure fit.
Hygienic Manufacturing
iCare probes are manufactured in a clean and controlled environment employing conditions of class 7 according to ISO 14644-01 and bioburden control of the environment (EU cGMP Annex 1; USP Chapter 116).
Our probes come individually packed in protective tubes, designed for single use. This minimizes the risk of microbiological cross-contamination and potential transmission of infectious diseases.